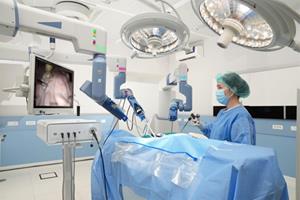
Press Release
Asensus Announces Senhance System Placement at GPR Klinikum Rüsselsheim of Germany
“With GPR’s broad laparoscopic use, the Senhance Surgical System is a perfect fit for high-volume surgical specialities like gynecology, urology and general surgery,” said
The Senhance Surgical System advances clinical intelligence by providing surgeons with augmented intelligence and digital tools to perform consistently superior surgery.
“We are proud to launch our Senhance surgical program and offer digital surgery to our patients,” said Mr.
About GPR Klinikum Rüsselsheim
About
Follow Asensus
Email Alerts: https://ir.asensus.com/email-alerts
LinkedIn: https://www.linkedin.com/company/asensus-surgical-inc
Twitter: https://twitter.com/AsensusSurgical
YouTube: https://www.youtube.com/c/transenterix
Vimeo: https://vimeo.com/asxc
Forward-Looking Statements
This press release includes statements relating to the Senhance Surgical System and GPR Gesundheits- und Pflegezentrum Klinikum of Russelsheim (“GPR”) initiating a program dedicated to pediatric procedures with the Senhance System. These statements and other statements regarding our future plans and goals constitute "forward looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations and include whether the Senhance Surgical System will be a perfect fit for high-volume surgical specialties like gynecology, urology and general surgery at GPR and whether the acceleration of the Senhance System’s traction in
INVESTOR CONTACT:
MEDIA CONTACT:
CG Life
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/da13399f-f61a-4e08-8b79-1c862dafb1a5

Source: Asensus Surgical, Inc.